Knowledge Translation
Our team synthesises, disseminates, reviews and exchanges evidence based ethically-sound knowledge to help improve the health and well being of those who suffer from all forms of allergies.

JACI In Practice
Management of Chronic Spontaneous Urticaria Made Practical: What Every Clinician Should Know
JACI In Practice
Management of Chronic Spontaneous Urticaria Made Practical: What Every Clinician Should Know
September 2, 2025
Despite its significant impact on quality of life, optimal management of chronic spontaneous urticaria remains challenging because of knowledge gaps regarding triggers, treatment response variability, and limited data for special populations. Second-generation H1-antihistamines are the first-line treatment and effective in approximately 50% of patients. For those who remain symptomatic, up-dosing up to fourfold is safe and recommended. However, prolonged ineffective antihistamine therapy should be avoided to prevent delayed disease control. In such cases, timely escalation to biologics, particularly omalizumab, is essential. Omalizumab remains the cornerstone of biologic therapy, offering rapid and sustained efficacy with an excellent safety profile. Personalized approaches involving dose escalation or interval adjustments further optimize outcomes. Cyclosporine A serves as an effective third-line option, particularly for autoimmune chronic spontaneous urticaria, but requires close monitoring because of dose-related adverse effects. Special considerations for children, pregnant individuals, and elderly people are discussed, reflecting the need for tailored approaches. Trigger avoidance, particularly nonsteroidal anti-inflammatory drugs, may aid management, although evidence is limited for many suspected exacerbating factors. Regular assessment of disease activity and control using validated tools such as the Urticaria Activity Score and Urticaria Control Test is essential for guiding treatment decisions and monitoring response. Updated international guidelines are anticipated to address emerging therapies and current knowledge gaps.
J Allergy Clin Immunol Pract. 2025 Sep;13(9):2252-2269. doi:
BMJ
Core GRADE 7: principles for moving from evidence to recommendations and decisions
BMJ
Core GRADE 7: principles for moving from evidence to recommendations and decisions
June 3, 2025
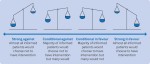
This seventh article in a seven part series presents the Core GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach for moving from evidence to recommendations or policy decisions. Core GRADE users make strong recommendations for an intervention versus a comparator when the desirable consequences clearly outweigh the undesirable consequences, and a conditional (weak) recommendation when the balance is less clear. Primary considerations in deciding on recommendations considering an individual patient perspective include balance of benefits, harms, and burdens; the certainty of evidence; and values and preferences. Secondary considerations, most important from a population perspective, include costs, feasibility, acceptability, and equity. Moving from evidence to recommendations begins with considering evidence regarding patients’ values and preferences and choosing the smallest difference in each outcome that patients perceive as important (the minimal important difference). Core GRADE users construct statements that make clear the values and preferences underlying their recommendations. In general, Core GRADE users make strong recommendations only when certainty of evidence is high or moderate. When evidence certainty is low, recommendations will be conditional under all but special circumstances.
BMJ. 2025 Jun 3;389:e083867. doi: 10.1136/bmj-2024-083867. PMID: 40461180.

BMJ
Core GRADE 6: presenting the evidence in summary of findings tables
BMJ
Core GRADE 6: presenting the evidence in summary of findings tables
May 27, 2025
This sixth article in a seven part series presents the Core GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to summary of findings tables. These tables provide essential information about the effects of interventions on patient important outcomes, including relative and absolute effects, certainty of evidence, and a plain language summary. For binary outcomes calculating absolute effects requires applying relative risk estimates to baseline risks from studies representative of the target population. For groups of patients with very different baseline risks, summary of findings tables include separate rows with different estimates of absolute effects. For continuous outcomes, challenges arise when individual studies use different instruments to measure patient reported outcomes. Facilitating interpretation then requires providing details about units of measurement and minimally important differences.
BMJ. 2025 May 27;389:e083866. doi: 10.1136/bmj-2024-083866. PMID: 40425239.
BMJ
Core GRADE 5: rating certainty of evidence-assessing indirectness
BMJ
Core GRADE 5: rating certainty of evidence-assessing indirectness
May 20, 2025
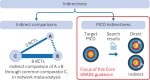
This fifth article in a seven part series presents the Core GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to systematic reviews, clinical practice guidelines, and health technology assessments and addresses issues of indirect evidence. Guideline developers and health technology assessment practitioners must carefully specify the population, intervention, comparison, and outcome (PICO)—their target PICO—and consider the extent to which the best available evidence matches their target. When target and study PICOs differ substantially, studies provide indirect evidence and Core GRADE users may rate down the certainty of evidence as a result of this indirectness. Whether examining studies from a search for direct evidence or a deliberate search for indirect evidence, for each substantial difference between target and study PICO Core GRADE users must judge the likelihood that magnitude of effects will differ substantially. The greater the likelihood of substantial differences the more advisable rating down for indirectness.
BMJ. 2025 May 20;389:e083865. doi: 10.1136/bmj-2024-083865. PMID: 40393729.
BMJ
Core GRADE 4: rating certainty of evidence-risk of bias, publication bias, and reasons for rating up certainty
BMJ
Core GRADE 4: rating certainty of evidence-risk of bias, publication bias, and reasons for rating up certainty
May 13, 2025
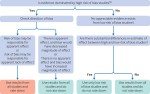
This fourth article in a seven part series presents the Core GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to addressing risk of bias, publication bias, and rating up certainty. In Core GRADE, randomised controlled trials begin as high certainty evidence and non-randomised studies of interventions (NRSI) as low certainty. To assess certainty of evidence for risk of bias, Core GRADE users first classify individual studies as low or high risk of bias. Decisions regarding rating down for risk of bias will depend on the weights of high and low risk of bias studies and similarities or differences between the results of high and low risk of bias studies. For publication bias, a body of evidence comprising small studies funded by industry should raise suspicion. Core GRADE users appraising results from well conducted NSRI can consider rating up certainty of evidence when risk ratios from pooled estimates suggest large or very large effects.
BMJ. 2025 May 13;389:e083864. doi: 10.1136/bmj-2024-083864. PMID: 40360206.
BMJ
Core GRADE 3: rating certainty of evidence-assessing inconsistency
BMJ
Core GRADE 3: rating certainty of evidence-assessing inconsistency
May 6, 2025
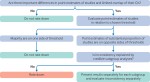
This third article in a seven part series presents the Core GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach to deciding whether to rate down certainty of evidence due to inconsistency—that is, unexplained variability in results across studies. For binary outcomes in which relative effects are consistent across baseline risks while absolute effects are not, Core Grade users assess consistency in relative effects. For continuous outcomes, they assess consistency in the absolute effects. When planning for the possibility of inconsistent results across studies, systematic review authors using Core GRADE construct a priori hypotheses regarding population or intervention characteristics that may explain inconsistency. They then judge the magnitude of inconsistency by considering the extent to which point estimates differ and the degree to which confidence intervals overlap. Before making a decision on rating down, Core GRADE users will evaluate where individual study estimates lie in relation to the threshold of the certainty rating (minimal important difference or the null). Finally, they will test their subgroup hypothesis and if an effect proves credible will provide separate evidence summaries and rate certainty of evidence separately for each subgroup. When they find no credible subgroup effect, they will provide a single evidence summary, rating down for inconsistency if necessary.
MJ. 2025 May 6;389:e081905. doi: 10.1136/bmj-2024-081905. PMID: 40328467.
BMJ
Core GRADE 1: overview of the Core GRADE approach
BMJ
Core GRADE 1: overview of the Core GRADE approach
April 22, 2025
This first article in a seven part series presents an overview of the essential elements of the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach that has proved extremely useful in systematic reviews, health technology assessment reports, and clinical practice guidelines. GRADE guidance has appeared in many articles dealing with both core issues and more specialised and complex guidance, and it has evolved over time. This series of articles presents GRADE essentials, Core GRADE, focusing on the core judgments necessary to summarise the comparative evidence about alternative care options and to make recommendations that apply to the care of individual patients. This article presents detailed guidance on formulating questions using the PICO (population, intervention, comparison, outcome) structure, and refining the question considering possible differences in relative and absolute effects across patient groups. The article then provides an overview of the remainder of the Core GRADE approach, including decisions about the certainty of the evidence and considerations in moving from evidence to guidance and recommendations.
BMJ. 2025 Apr 22;389:e081903. doi: 10.1136/bmj-2024-081903. PMID: 40262844.

WAO
Time to ACT-UP: Update on precautionary allergen labelling (PAL)
WAO
Time to ACT-UP: Update on precautionary allergen labelling (PAL)
September 25, 2024
Background: Precautionary Allergen ("may contain") Labelling (PAL) is used by industry to communicate potential risk to food-allergic individuals posed by unintended allergen presence (UAP). In 2014, the World Allergy Organization (WAO) highlighted that PAL use was increasing, but often applied inconsistently and without regulation - which reduces its usefulness to consumers with food allergy and those purchasing food for them. WAO proposed the need for a regulated, international framework to underpin application of PAL. In 2019, the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations convened an expert consultation to address the issue of PAL, the outputs of which are now being considered by the Codex Committee on Food Labelling (CCFL).
Objectives: To summarise the latest data to inform the application of PAL in a more systematic way, for implementation into global food standards.
Methods: A non-systematic review of issues surrounding precautionary labelling and food allergens in pre-packaged products.
Results: Approximately, 100 countries around the world have legislation on the declaration of allergenic ingredients. Just a few have legislation on UAP. Given the risks that UAP entails, non-regulated PAL creates inconvenience in real life due to its unequal, difficult interpretation by patients. The attempts made so far to rationalize PAL present lights and shadows.
Conclusions: At a time when CCFL is considering the results of the FAO/WHO Expert Consultation 2020-2023, we summarise the prospects to develop an effective and homogeneous legislation at a global level, and the areas of uncertainty that might hinder international agreement on a regulated framework for PAL of food allergens.
Objectives: To summarise the latest data to inform the application of PAL in a more systematic way, for implementation into global food standards.
Methods: A non-systematic review of issues surrounding precautionary labelling and food allergens in pre-packaged products.
Results: Approximately, 100 countries around the world have legislation on the declaration of allergenic ingredients. Just a few have legislation on UAP. Given the risks that UAP entails, non-regulated PAL creates inconvenience in real life due to its unequal, difficult interpretation by patients. The attempts made so far to rationalize PAL present lights and shadows.
Conclusions: At a time when CCFL is considering the results of the FAO/WHO Expert Consultation 2020-2023, we summarise the prospects to develop an effective and homogeneous legislation at a global level, and the areas of uncertainty that might hinder international agreement on a regulated framework for PAL of food allergens.
World Allergy Organ J . 2024 Sep 25;17(10):100972. doi: 10.1016/j.waojou.2024.100972.

JACI In Practice
Patient-Reported Outcome Measures in Chronic Spontaneous Urticaria, Angioedema, and Atopic Dermatitis
JACI In Practice
Patient-Reported Outcome Measures in Chronic Spontaneous Urticaria, Angioedema, and Atopic Dermatitis
August 19, 2024
Reducing the burden of disease for patients and families requires being able to measure health status changes related to disease severity, control, and response to treatment over time. Patient-reported outcomes are patient perceptions of their health status. Such perceptions are critical to decision making. Some patient-reported outcome measures (PROMs) are extensive and often intended to be used only for detailed research assessments. Many PROMs, however, form critical components of valid, reliable, and responsive assessments in clinical research and routine clinical practice. The smallest score change in a PROM that would lead to different decision making by patients is called the minimally important difference. Using PROMs may also offer advantages over general questions or unvalidated tools. With the innovation of technology, the ability to chronicle disease symptoms using communication technology (mobile phone applications) has become increasingly available. Collection of real-world data in this capacity will be very useful for identifying more precise phenotypes/endotypes necessary for investigation of tailored therapies for chronic spontaneous and inducible urticaria, angioedema, and atopic dermatitis. Here, we provide an overview of PROMs that have been developed for the assessment of disease severity, control, and quality of life and that have been validated for the use of adults and children with these skin disorders.
J Allergy Clin Immunol Pract. 2024 Oct;12(10):2583-2590. doi: 10.1016/j.jaip.2024.08.021. Epub 2024 Aug 19. PMID: 39168302.
The Dermatology Digest
The Itch Factor Unpacking the New AAAAI/ACAAI Joint Task Force Guidelines on AD
The Dermatology Digest
The Itch Factor Unpacking the New AAAAI/ACAAI Joint Task Force Guidelines on AD
March 14, 2024
Following a prolonged period of little progress, there has been a surge in the approval of new treatments for atopic dermatitis (AD), with numerous others in different stages of development. For healthcare professionals treating AD, the abundance of options can be daunting, posing challenges to effectively incorporating these new therapies into patient care. The American Academy of Allergy, Asthma & Immunology (AAAAI) and the American College of Allergy, Asthma & Immunology (ACAAI) Joint Task Force (JTF) recently released updated guidelines for AD management, encompassing several of the latest medications and addressing five crucial questions related to AD management:
The Dermatology Digest

JACI In Practice
Impacts of Determinants of Health on Atopic Dermatitis, Pathways Towards Solutions, and Unique Considerations for Rural and Remote North American Indigenous Populations
JACI In Practice
Impacts of Determinants of Health on Atopic Dermatitis, Pathways Towards Solutions, and Unique Considerations for Rural and Remote North American Indigenous Populations
November 24, 2023
Disparities in environmental and social determinants of health (DOH) are associated with morbidity in atopic dermatitis (AD). The socioecological model (SEM) is a framework that can be applied to better understand how DOH impacts patients with AD. We include a case scenario of a remote Indigenous patient reflective of real-world situations of living with AD and examine relevant impact, gaps in knowledge, and further research needs. This review highlights a variety of social and environmental exposures as important DOH which must be addressed to achieve optimal management in AD. The "Rainbow model” is a modified framework to help illustrate how complex environmental and social forces impact both AD presentation and therapeutic success. However, practical applications and outcome metrics for health promotion are limited.1 An inter- and transdisciplinary approach is paramount to address the complex challenges associated with AD care, as well as multistakeholder approach integrating culturally-competent equitable health frameworks. This review underscores the importance of expanding the focus of AD management beyond basic science and clinical trials to recognize and address health disparities and to promote optimal health and well-being in patients with AD, and contributes a working approach to mapping the complex interventions and patient-oriented research needed using a focus on remote North American Indigenous patients affected by AD.
In Press

BMJ
A guide and pragmatic considerations for applying GRADE to network meta-analysis
BMJ
A guide and pragmatic considerations for applying GRADE to network meta-analysis
June 27, 2023
Assessing the certainty of evidence from network meta-analyses using the GRADE (grading of recommendations, assessment, development, and evaluations) approach requires not only a thorough understanding of the methods but also substantial workload for raters. This article describes how implementing practical strategies (including rule setting and automation) can facilitate efficient application of the GRADE approach to rating certainty of evidence in network meta-analyses while maintaining rigor.
BMJ 2023;381:e074495

JACI In Practice
Skin antiseptics for atopic dermatitis: dissecting facts from fiction
JACI In Practice
Skin antiseptics for atopic dermatitis: dissecting facts from fiction
January 22, 2023
Staphylococcus aureus (S. aureus) is a known trigger and cause of infectious complications in atopic dermatitis (AD). Various antiseptics have been used in an attempt to decrease the burden of S. aureus in AD. In this Commentary, we present the evidence for and against some of the commonly-used antiseptics in clinical and research settings. These agents remain attractive as an adjunct therapy for AD due to their relative low cost and potential benefits of reducing S. aureus. Though a number of studies have evaluated the use of dilute bleach, its mechanisms remain controversial. A higher concentration of bleach than the commonly-used 0.005% is likely needed for its anti-S. aureus effect. Silver-coated textiles have demonstrated anti-S. aureus effects in various studies, however, their efficacy and side effects in AD remain to be confirmed. Other antiseptics including chlorhexidine, triclosan and triclocarban are also discussed. Variables that may affect the outcomes of these studies include length of use, concurrent application of moisturizers and anti-inflammatory medications.
Ong, PY, Boguniewicz, J, Chu DK. The Journal of Allergy and Clinical Immunology: In Practice, In Press

Canadian Allergy & Immunology Today
Atopic Dermatitis and Canadian Indigenous Peoples: Burdens, Barriers, and Potential for Solutions
Canadian Allergy & Immunology Today
Atopic Dermatitis and Canadian Indigenous Peoples: Burdens, Barriers, and Potential for Solutions
December 1, 2022
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease worldwide. AD begins before age five in 90% of cases and is associated with the development of comorbid conditions such as infections, environmental allergies, food allergies, asthma, anxiety/depression, and abnormalities in sleep, growth, and development. While personal experience suggests that Canadian Indigenous peoples, including children and youth, are facing burdens associated with AD, formal research studies addressing the impact of AD and skin disease in general on Canadian Indigenous peoples are lacking. Canadian Indigenous* account for approximately 5% of the Canadian population. Although Canadian Indigenous vary widely in geography, culture, language, and beliefs, they face common health disparities embedded in complex historical and social contexts related to factors such as colonization, intergenerational trauma from residential schools and institutionalization, racial segregation in the form of reservations, systemic racism, and being subjected to policies such as the ‘Indian Act’. This article reviews the burdens and barriers of AD in Canadian Indigenous by examining the literature, experiences of health care practitioners (HCPs), and media reports followed by proposing potential solutions to address such disparities.
Can Allergy Immunol Today. 2022 Dec. 1;2(3):26–30.

Canadian Medical Association Journal (CMAJ)
How to manage atopic dermatitis in infants
Canadian Medical Association Journal (CMAJ)
How to manage atopic dermatitis in infants
November 7, 2022
Atopic dermatitis affects an 10-20% of Canadian infants and negatively impacts patient and caregiver quality of life in many ways. Despite this, optimal treatment is challenging, with caregivers often leaving clinical encounters unequipped to gain self-efficacy and clinicians uncertain of which treatments to promote or demote. There is also increasing evidence for addressing comorbidity in atopic dermatitis, particularly its role in promoting the development of food allergy and the importance, for infants, of timing of food allergen introduction in food allergy prevention. To address these issues, we summarize five key messages for general and specialist care providers as well as caregivers to better understand how to manage atopic dermatitis.
CMAJ November 07, 2022 194 (43) E1485
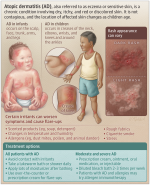
JAMA Pediatrics
What Parents Should Know About Atopic Dermatitis
JAMA Pediatrics
What Parents Should Know About Atopic Dermatitis
September 26, 2022
Atopic dermatitis (AD) is a chronic skin condition that results in dry, itchy, and red skin.
More than 1 in 10 children are affected. It is caused by dysfunction of the skin barrier and an imbalanced immune system. Children with AD are often described as having sensitive skin.
More than 1 in 10 children are affected. It is caused by dysfunction of the skin barrier and an imbalanced immune system. Children with AD are often described as having sensitive skin.
JAMA Pediatr. doi:10.1001/jamapediatrics.2022.3109

JACI In Practice
Boxed warnings and off-label use of allergy medications: risks, benefits, and shared decision making
JACI In Practice
Boxed warnings and off-label use of allergy medications: risks, benefits, and shared decision making
September 6, 2022
The Food and Drug Administration (FDA) is tasked with evaluating the efficacy and safety of a drug. Despite having a regimented appraisal process in place, safety evidence can emerge during clinical trials as well as from observations and studies conducted after the drug has been on the market that might require a boxed warning. The boxed warning is the most severe warning that the FDA can give to an approved drug. It is commonly referred to as a Black Box Warning as it is outlined in the package insert by a thick black box to garner the attention of prescribers and patients. There are currently over 400 medications that have boxed warnings and the information addressing major risks associated with a particular drug may, appropriately or inappropriately, influence patient and clinician decision making. Healthcare professionals must use the best evidence and clinical judgment in determining whether to prescribe medications with these warnings. Use of an approved drug at dosages or for indications other than what it was originally licensed for is referred to as “off-label” and is legal, commonplace, and may be evidence-based. All drugs may expose patients to possible harm, so prescribers have an obligation to discuss the best available evidence regarding benefits and harms so that patients can participate in shared decision-making.
The Journal of Allergy and Clinical Immunology: In Practice (2022), doi: https://doi.org/10.1016/ j.jaip.2022.08.033.

World Allergy Organization
Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines update - III - Cow's milk allergens and mechanisms triggering immune activation
World Allergy Organization
Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines update - III - Cow's milk allergens and mechanisms triggering immune activation
September 1, 2022
The immunopathogenesis of cow's milk protein allergy (CMPA) is based on different mechanisms related to immune recognition of protein epitopes, which are affected by industrial processing.
Purpose
The purpose of this WAO DRACMA paper is to: (i) give a comprehensive overview of milk protein allergens, (ii) to review their immunogenicity and allergenicity in the context of industrial processing, and (iii) to review the milk-related immune mechanisms triggering IgE-mediated immediate type hypersensitivity reactions, mixed reactions and non-IgE mediated hypersensitivities.
Results
The main cow’s milk allergens – α-lactalbumin, β-lactoglobulin, serum albumin, caseins, bovine serum albumins, and others – may determine allergic reactions through a range of mechanisms. All marketed milk and milk products have undergone industrial processing that involves heating, filtration, and defatting. Milk processing results in structural changes of immunomodulatory proteins, leads to a loss of lipophilic compounds in the matrix, and hence to a higher allergenicity of industrially processed milk products. Thereby, the tolerogenic capacity of raw farm milk, associated with the whey proteins α-lactalbumin and β-lactoglobulin and their lipophilic ligands, is lost.
Conclusion
The spectrum of immunopathogenic mechanisms underlying cow's milk allergy (CMA) is wide. Unprocessed, fresh cow's milk, like human breast milk, contains various tolerogenic factors that are impaired by industrial processing. Further studies focusing on the immunological consequences of milk processing are warranted to understand on a molecular basis to what extent processing procedures make single milk compounds into allergens.
Purpose
The purpose of this WAO DRACMA paper is to: (i) give a comprehensive overview of milk protein allergens, (ii) to review their immunogenicity and allergenicity in the context of industrial processing, and (iii) to review the milk-related immune mechanisms triggering IgE-mediated immediate type hypersensitivity reactions, mixed reactions and non-IgE mediated hypersensitivities.
Results
The main cow’s milk allergens – α-lactalbumin, β-lactoglobulin, serum albumin, caseins, bovine serum albumins, and others – may determine allergic reactions through a range of mechanisms. All marketed milk and milk products have undergone industrial processing that involves heating, filtration, and defatting. Milk processing results in structural changes of immunomodulatory proteins, leads to a loss of lipophilic compounds in the matrix, and hence to a higher allergenicity of industrially processed milk products. Thereby, the tolerogenic capacity of raw farm milk, associated with the whey proteins α-lactalbumin and β-lactoglobulin and their lipophilic ligands, is lost.
Conclusion
The spectrum of immunopathogenic mechanisms underlying cow's milk allergy (CMA) is wide. Unprocessed, fresh cow's milk, like human breast milk, contains various tolerogenic factors that are impaired by industrial processing. Further studies focusing on the immunological consequences of milk processing are warranted to understand on a molecular basis to what extent processing procedures make single milk compounds into allergens.
World Allergy Organization Journal Volume 15, Issue 9, September 2022, 100668

Trustworthy Patient-Centered Guidelines: Insights From Atopic Dermatitis and a Proposal for the Future
Trustworthy Patient-Centered Guidelines: Insights From Atopic Dermatitis and a Proposal for the Future
July 7, 2022
Not all clinical practice guidelines are trustworthy. A proliferation of guidelines, many with suboptimal rigor and transparency, led the Institute of Medicine to create standards defining objective and consistent guideline development processes. Trustworthy guidelines optimize care by issuing systematically developed statements to assist practitioner and patient decisions addressing optimal health care for specific clinical circumstances. To be trustworthy, guidelines should follow systematic and rigorous development processes, apply the principles of evidence-based medicine, and place patient and caregiver values and preferences as central to optimal decision making.
The Journal of Allergy and Clinical Immunology: In Practice, https://doi.org/10.1016/j.jaip.2022.06.017

JACI In Practice
Translating Evidence to Optimize Patient Care Using GRADE
JACI In Practice
Translating Evidence to Optimize Patient Care Using GRADE
December 1, 2021
Optimal evidence-based clinical practice requires systematic summaries of the best available evidence, including ratings of the quality of that evidence, and is facilitated by the availability of trustworthy guidelines. In this review, we describe the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to rating quality of evidence and moving from evidence to recommendations using examples from allergy-immunology. GRADE focuses on systematic summaries of the best evidence, systematic reviews and trustworthy guidelines, and emphasizes a structured approach to determining quality (certainty) of bodies of evidence, absolute magnitude of effects of desirable and undesirable consequences (benefits and harms), and use of evidence to develop clinical recommendations. Adopted by over 110 organizations worldwide, including the American Academy of Allergy, Asthma, and Immunology/American College of Allergy, Asthma, and Immunology Joint Task Force on Practice Parameters, GRADE is foundational to the optimal interpretation of research evidence and its application in clinical practice. This review supports the clinician's ability to find and use the information in GRADE guidelines to help care for patients in the clinic.
The Journal of Allergy and Clinical Immunology: In Practice, Volume 9, Issue 12, 2021, Pages 4221-4230

Pediatric Allergy and Immunology
Method’s corner: Allergist’s guide to network meta-analysis
Pediatric Allergy and Immunology
Method’s corner: Allergist’s guide to network meta-analysis
July 29, 2021
Network meta-analyses (NMAs) simultaneously estimate the effects of multiple possible treatment options for a given clinical presentation. For allergists to benefit optimally from NMAs, they must understand the process and be able to interpret the results. Through a worked example published in Pediatric Allergy and Immunology, we summarize how to identify credible NMAs and interpret them with a focus on recent innovations in the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation). NMAs build on traditional systematic reviews and meta-analyses that consider only direct paired comparisons by including indirect evidence, thus allowing the simultaneous assessment of the relative effect of all pairs of competing alternatives. Our framework informs clinicians of how to identify credible NMAs and address the certainty of the evidence. Trustworthy NMAs fill a critical gap in providing key inferences using direct and indirect evidence to inform clinical decision making when faced with more than two competing courses of treatment options. This document will help allergists to identify trustworthy NMAs to enhance patient care.
Pediatr Allergy Immunol. 2021; 33:e13609.

Lancet Respiratory Medicine
Use of facemasks during the COVID-19 pandemic
Lancet Respiratory Medicine
Use of facemasks during the COVID-19 pandemic
August 3, 2020
As of July 26, 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 16 000 000 individuals worldwide and caused over 600 000 deaths from COVID-19. Despite advances in pharmacological treatment and early vaccine development, reducing transmission of the virus with the use of facemasks (referring to medical or surgical masks, N-95 and similar respirators, cloth masks, and bandannas) by health-care workers and the public alike remains a hotly debated topic due to politicisation of discourse and decision making.

Annals of Internal Medicine
Rethinking Oxygen Therapy for Hospitalized Patients
Annals of Internal Medicine
Rethinking Oxygen Therapy for Hospitalized Patients
September 17, 2019
Oxygen therapy has been a critical life-saving measure since its first use for pneumonia in the 19th century. With its widespread use in contemporary medicine came the notion that supplemental oxygen is harmless, and that it may even be beneficial in nonhypoxemic patients. Over time, this conviction has become ingrained in clinical practice and the medical training of health care providers. Indeed, the classic mantra taught for any acutely ill patient is, “ABC IV O2 Monitor.”
Ann Intern Med.2019;171:HO2-HO3.

The Intern at Work
Podcast for Internal Medicine - Penicillin allergy “Rash Decisions”
The Intern at Work
Podcast for Internal Medicine - Penicillin allergy “Rash Decisions”
April 14, 2019
Penicillin Allergy
Ask a Fellow: Rash Decisions - Penicillin Allergy
Guest Fellows: Dr. Derek Chu (Clinical Allergy and Immunology), Dr. David McCullagh (Infectious Diseases)
Infographic by: Rachel Ahle (Physician Assistant Student)
Ask a Fellow: Rash Decisions - Penicillin Allergy
Guest Fellows: Dr. Derek Chu (Clinical Allergy and Immunology), Dr. David McCullagh (Infectious Diseases)
Infographic by: Rachel Ahle (Physician Assistant Student)

AAAAI
Food Labels: Read It Before You Eat It!
AAAAI
Food Labels: Read It Before You Eat It!
January 16, 2019
For people with food allergies, food allergen avoidance is a critical part of preventing allergic reactions. Understanding how to read a food label is necessary to effectively avoid any food to which one might be allergic.
Reading a food label for allergens is different from what you might be used to. It is more than just looking at the carbs, protein, salt and calorie count. Instead, for food allergies, the ingredient list and any warning labels are the most important. However, packaged and processed foods often contain many ingredients and sometimes they are not labelled in a straightforward way. This can make reading food ingredient labels difficult and it may be hard to know how a particular ingredient relates to your allergy.
Here are a few tips and things to keep in mind when reading a food label for food allergy:
Reading a food label for allergens is different from what you might be used to. It is more than just looking at the carbs, protein, salt and calorie count. Instead, for food allergies, the ingredient list and any warning labels are the most important. However, packaged and processed foods often contain many ingredients and sometimes they are not labelled in a straightforward way. This can make reading food ingredient labels difficult and it may be hard to know how a particular ingredient relates to your allergy.
Here are a few tips and things to keep in mind when reading a food label for food allergy:




